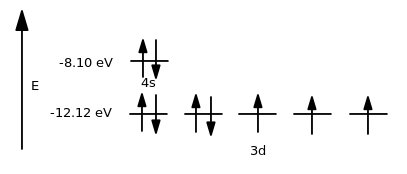
It is the first #4s# electron chosen.
In cobalt, the higher-energy orbital when comparing the #4s# and the #3d# subshells is the #\mathbf(4s)# orbital. In fact, the #4s# orbital is about #"4.02 eV"# higher in energy.
 )
)
The first electron removed from the #4s# orbital, which is doubly-occupied, is easy to remove because it is paired.
The electron pairing adds charge repulsion between the electrons, increasing the ease of removing the first one.
The second one in the #4s# orbital (after removing the first one already) is a bit harder to remove because it doesn't have the repulsion energy that it did when the electrons were paired.
Consider the ionization energies of the first two electrons:
First ionization energy: #"7.881 eV" ("760.41 kJ/mol")# (removing first electron)
Second ionization energy: #"17.08 eV" ("1648.27 kJ/mol")# (removing BOTH electrons)
Thus, the ionization energy for removing the second electron is #17.08 - 7.881 = "9.20 eV"#, which is #"1.32 eV" ("127.36 kJ/mol")# larger, and confirms that repulsion energy plays a role in the ease of removing the first electron relative to the second.
 )
) 
