Question #ce7a4
1 Answer
Explanation:
As it turns out, the problem already provides you with the molecular formula and the empirical formula of aspirin, or acetylsalicyclic acid,
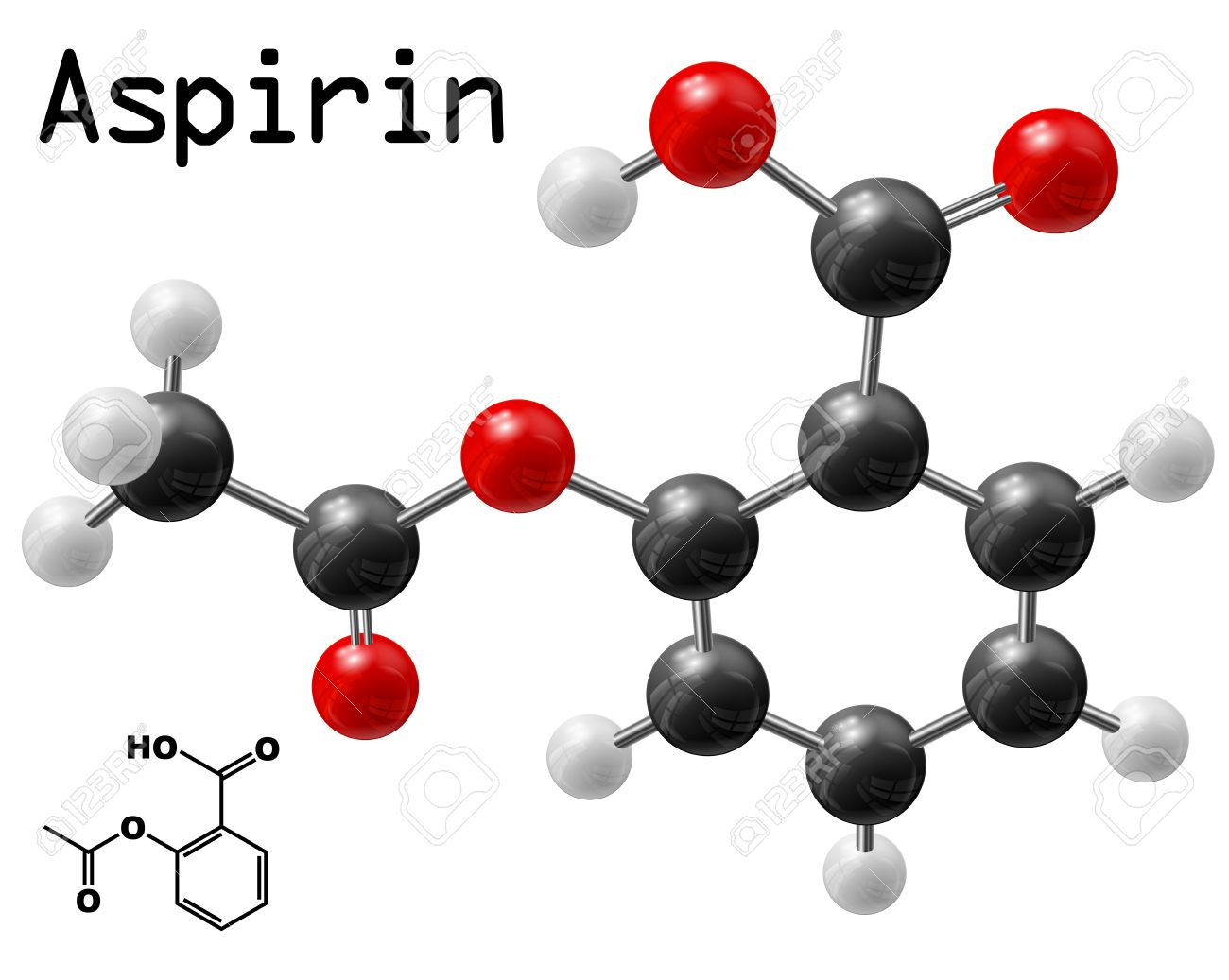
A compound's molecular formula tells you the exact number of atoms of each constituent element present in one molecule of this compound. In your case, one molecule of aspirin contains
- nine atoms of carbon, shown here in black
- eight atoms of hydrogen, shown here in light gray
- four atoms of oxygen, shown here in red
This is why the compound's molecular formula is
Now, the empirical formula tells you the smallest whole number ratio that exists between a compound's constituent elements.
In this case, a
#color(green)(|bar(ul(color(white)(a/a)color(black)("C"_9"H"_8"O"_4)color(white)(a/a)|))) -># empirical and molecular formula

