You're essentially dealing with four ionic compounds that are insoluble in aqueous solution.
Silver chloride, #"AgCl"#, is a white precipitate that can be obtained by mixing a solution of a soluble ionic compound that contains the silver(I) cation, #"Ag"^(+)#, with a solution of a soluble ionic compound that contains the chloride anion, #"Cl"^(-)#.
 )
)
Lead(II) chromate, #"PbCrO"_4#, is a yellow precipitate that can be obtained by mixing a solution of a soluble ionic compound that contains the lead(II) cation, #"Pb"^(2+)#, with a solution of a soluble ionic compound that contains the chromate anion, #"CrO"_4^(2-)#.
 )
)
Copper(II) sulfide, #"CuS"#, is a black precipitate that can be formed by mixing a solution of a soluble ionic compound that contains the copper(II) cation, #"Cu"^(2+)#, with a solution of a soluble ionic compound that contains the sulfide anion, #"S"^(2-)#.
 )
)
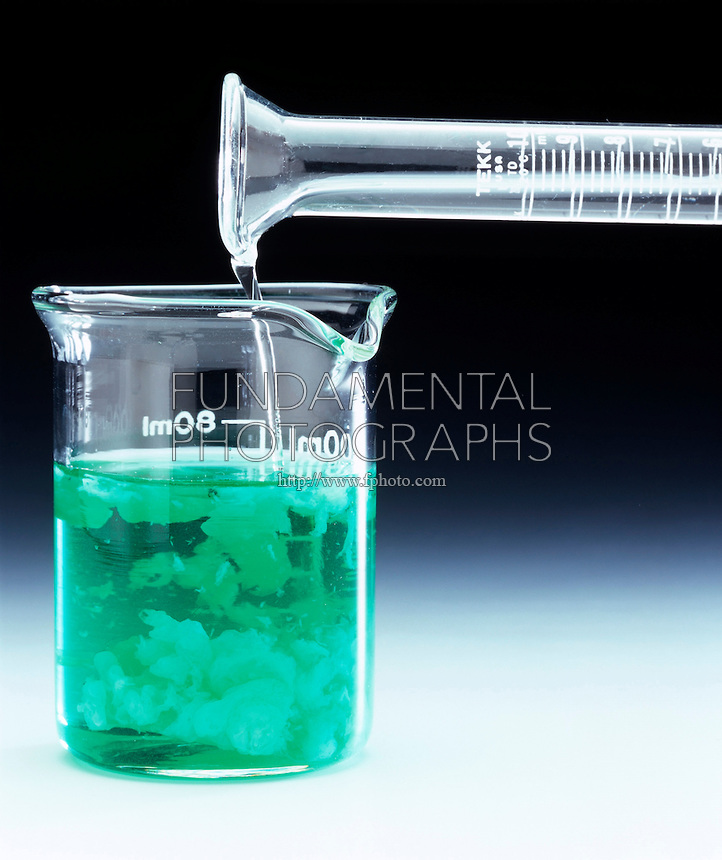
Finally, nickel(II) hydroxide, #"Ni"("OH")_2#, is a green precipitate that results when a solution of a soluble ionic compound that contains the nickel(II) cation, #"Ni"^(2+)#, is mixed with a solution of a soluble ionic compound that contains the hydroxide anion, #"OH"^(-)#.
 )
)
)
 )
)  )
)  )
) 
