Question #fc782
1 Answer
Explanation:
The idea here is that you need to use the masses of the two elements you get in the first sample to find the mole ratio that exists between carbon and oxygen in ascorbic acid.
This will then allow you to find the mass of oxygen that would correspond to that many grams of carbon in the second sample.
So, use the molar masses of the two elements to find how many moles of each you get in the first sample
#"For C: " 1.50color(red)(cancel(color(black)("g"))) * "1 mole C"/(12.011color(red)(cancel(color(black)("g")))) = "0.1249 moles C"#
#"For O: " 2.00color(red)(cancel(color(black)("g"))) * "1 g O"/(15.9994color(red)(cancel(color(black)("g")))) = "0.1250 moles O"#
As you can see, the two elements exist in a
#"For C: " (0.1249color(red)(cancel(color(black)("moles"))))/(0.1249color(red)(cancel(color(black)("moles")))) = 1#
#"For O: " (0.1250color(red)(cancel(color(black)("moles"))))/(0.1249color(red)(cancel(color(black)("moles")))) = 1.001 ~~ 1#
This means that every sample of ascorbic acid will contain equal numbers of moles of carbon and of oxygen.
The number of moles of carbon present in the second sample will be equal to
#6.35color(red)(cancel(color(black)("g"))) * "1 mole C"/(12.011color(red)(cancel(color(black)("g")))) = "0.5287 moles C"#
This means that the second sample must also contain
#0.5287color(red)(cancel(color(black)("moles O"))) * "15.9994 g"/(1color(red)(cancel(color(black)("mole O")))) = "8.4589 g"#
Rounded to three sig figs, the answer will be
#m_O = color(green)(|bar(ul(color(white)(a/a)"8.46 g oxygen"color(white)(a/a)|)))#
Indeed, a quick look at a molecule of ascorbic acid,
This confirms the
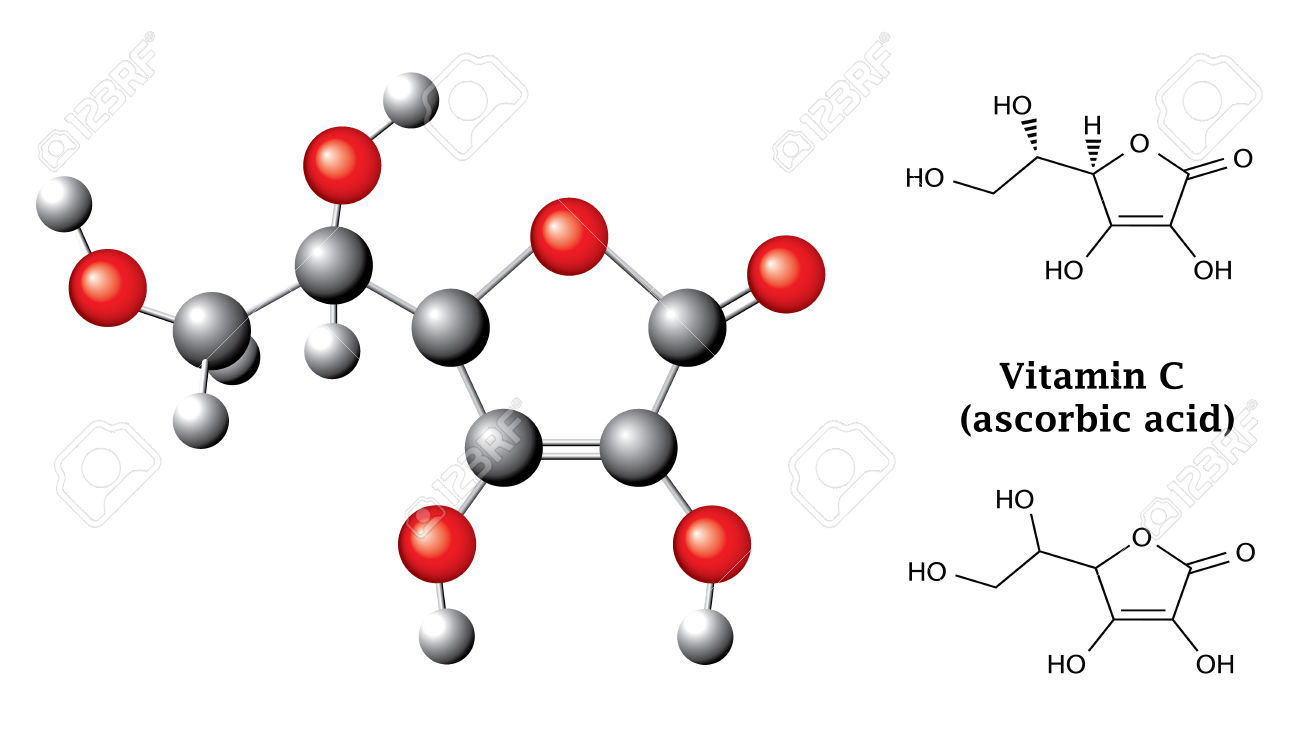
Here carbon atoms are shown in dark grey and oxygen atoms in red.

