Question #62993
1 Answer
Yes, we can.
Explanation:
You're dealing with the hydrolisis of boron trichloride,
This reaction will produce boric acid, which you'll see written both as
The balanced chemical equation would indeed look like this
#"BCl"_text(3(aq]) + 3"H"_2"O"_text((l]) -> "B"("OH")_text(3(aq]) + 3"HCl"_text((aq])#
I'll try to explain what's going on here without going into too much detail. Mind you, this is a simplified version of what actually goes on, but it will give you an idea of how things work here.
The boron atom in boron trichloride, which is
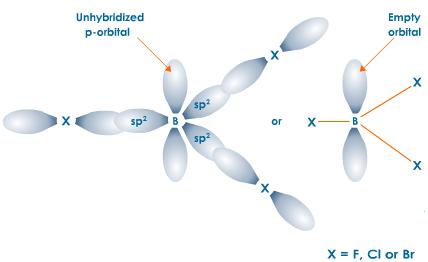
This means that it can act as a Lewis acid, i.e .accept a pair of electrons from the oxygen atom of a water molecule.
This will lead to the formation of an adduct, which is simply the result of the addition of two molecules.
Once this adduct, which can be represented as
This will trigger the replacement of the
![
)
As an interest follow-up, boric acid acts as an acid not because it donates protons,
#"B"("OH")_text(3(aq]) + "H"_2"O"_text((l]) rightleftharpoons ["B"("OH")_text(4(aq])]^(-) + "H"_text((aq])^(+)#

