Question #76806
1 Answer
Because of the lone pairs of electrons present on the oxygen atom.
Explanation:
Oxygen difluoride,
This molecular geometry ensures that the dipole moments associated with the oxygen - fluoride bonds do not cancel each other out to produce a nonpolar molecule.
To see why this is the case, draw the molecule's Lewis structure. The molecule will have a total of
#6# from the oxygen atom#7# from each of the two fluorine atoms
The oxygen atom will take the role of central atom, forming single bonds with the two fluoride atoms. These bonds will account for
The resulting
- three lone pairs on each fluorine atom
- two lone pairs on the oxygen atom
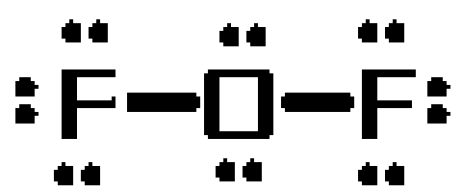
Now, it is very important to realize the Lewis structures are not meant to to convey molecular geometry!
In order to find the molecule's geometry, you count the regions of electron density that surround the central atom - these will give you the atom's steric number.
Regions of electron density are bonds to other atoms (here single, double, or triple bonds count as one region) and lone pairs of electrons.
In your case, the central oxygen atom is bonded to two other atoms and is surrounded by two lone pairs
According to VSEPR Theory, this corresponds to an

Now, the difference in electronegativity between fluorine and oxygen ensures that the two
The result will be the formation of a permanent dipole moment, and thus a polar molecule


