Question #0f813
1 Answer
Metals form cations.
Explanation:
Metal atoms lose one or more valence electrons when forming ions. The atoms of the alkali metals (group 1) have 1 valence electron, which they lose when forming compounds. The atoms of the alkaline earth metals (group 2) have 2 valence electrons, which they lose when forming compounds. Aluminum atoms, in group 13, have 3 valence electrons, which they lose when forming compounds.
The transition metals also lose electrons when forming compounds, and many if not most form more than one ion, such as copper, which forms
Alkali Metal Sodium and Nonmetal Chlorine
![http://cnx.org/contents/9e56ee2c-0f9c-4266-8197-fff3de034aa8@4.3:5/Chemistry-Grade-10-[CAPS]](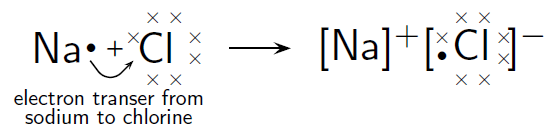 )
)
Alkaline Earth Metal Magnesium and Nonmetal Oxygen
![http://cnx.org/contents/9e56ee2c-0f9c-4266-8197-fff3de034aa8@4.3:5/Chemistry-Grade-10-[CAPS]](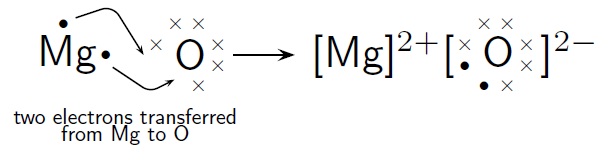 )
)
Aluminum and Oxygen

