Technically you would look at one atom as the central atom, and so you could choose an atom on the molecule to use as your central atom.
But cubane is weird. It has all #90^@# angles and is highly-strained, yet it is kinetically stable, and it doesn't readily decompose.
 )
)
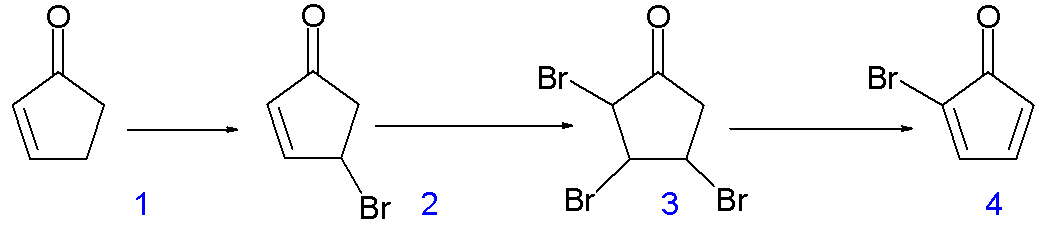
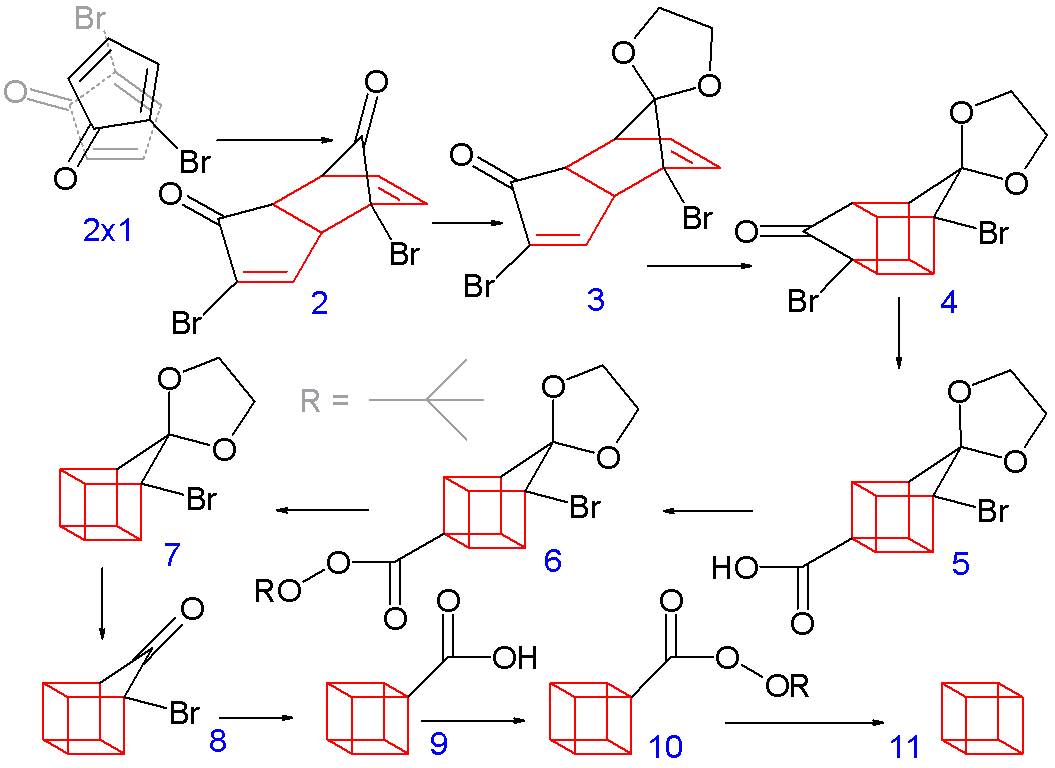
The synthesis is quite cool too.
 )
)
 )
)
Probably the main reason why it has many #90^@# angles is because of its three-dimensional bonding.
If you examined each corner of the cube in isolation, it should be tetrahedral, but in context with the other 7 corners, it becomes more like a derivative of the octahedral geometry (many #90^@# angles) because it would yield the least strain for the #"C"-"C"-"C"# angles to be #90^@# than for them to be closer to #109.5^@#.
You can tell that if you tried to increase the #"C"-"C"-"C"# bond angles (the corner angles), you would have to push in on the corners of cubane (as opposed to squishing them together, making the faces concave-inwards), which as you might imagine, puts even more strain on the bonds (which is not good).
It's different though for the #"C"-"C"-"H"# bond angles; the hydrogens have room for free rotation outside of the cube to minimize electron/electron repulsions, so they do so to minimize the ground-state energy of the molecule. I don't know what this additional bond angle is, but it's greater than #90^@# as you can tell from the structure.
)
 )
)  )
) 
