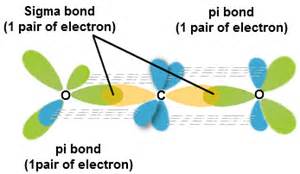
How do we describe the hybridization in the carbon dioxide molecule?
1 Answer
The bonds in carbon dioxide,
Explanation:
In terms of a valence bond description the carbon is