Calculate the enthalpy of reaction for the reaction #"CH"_3"COOH" + "H"_2"O" -> "CH"_3"CH"_2"OH" + "O"_2#?
2 Answers
Well, you could just look up the standard enthalpies of formation, although you didn't specify at what temperature or pressure, and what phases. My assumption though is that you're at STP.
 )
)
Acetic acid is here:
https://en.wikipedia.org/wiki/Acetic_acid_%28data_page%29
Its liquid
Its gaseous
I know that the ethanol is a liquid at STP, and oxygen is a gas at STP (it's also in its elemental state, so its enthalpy of formation is 0). Water might be a gas, or a liquid, but you have to know which one. Usually if it's a product, it's ambiguous without specifying...
I'll just give all four possibilities.
where
1)
2)
3)
4)
And then, considering how there is no
The accepted answer is
You can find the enthalpy change for your reaction by using bond enthalpies.
Explanation:
The enthalpy change for a reaction can also be calculate by using bond enthalpies. In any chemical reaction, some bonds are being broken and others are being formed.
More specifically, the bonds of the reactants are being broken and the bonds of the products are being formed.
The enthalpy change of the reaction will be the difference between the energy that's needed to break the bonds of the reactants and the energy that's given off when the bonds of the products are formed.
In order to get an idea about what bonds are being broken and what bonds are being formed, take a look at the Lewis structures of the specis that take part in th reaction
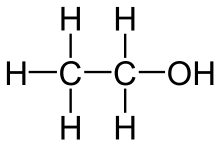
So, for ethanol, you need to break
- five
#"C"-"H"# bonds; - one
#"C"-"C"# bond; - one
#"C"-"O"# bond; - one
#"O"-"H"# bond.
For diatomic oxygen, you just need to break one
 )
)
For acetic acid, you need to form
- three
#"C"-"H"# bonds; - one
#"C"-"C"# bond; - one
#"C"-"O"# bond; - one
#"O"-"H"# bond; - one
#"C"="O"# bond.
For water, you need to form two
Here's a table containing the bond enthalpies you need for your reaction.
![http://www.kentchemistry.com/links/Kinetics/BondEnergy.htm]()
Since you're dealing with 1 mole of each compound, you can go ahead and use the bond enthlpies in kJ, rather than kJ per mole. So, the enthalpy change for your reaction will be
The accepted value for the enthalpy change of this reaction is actually -494 kJ, so double-check my calculations!


