How many valence electrons does zinc have?
1 Answer
You take a look at its electron configuration.
Explanation:
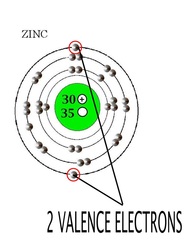
Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30.
This means that neutral zinc atom has a total of 30 electrons surrounding its nucleus. To see how many of these electrons are valence electrons, write the electron configuration of a neutral zinc atom.
or, if you use the noble gas shorthand notation
Notice that the outermost shell, which for zinc is the fourth shell,
This means that zinc can lose the two electrons located in the 4s-orbital to be become the
Notice that the 3d-orbital is completely filled, which means that these electrons behave like core electrons and cannot be considered valence electrons.
Therefore, zinc has only 2 valence electrons, both located in the 4s-orbital.