Question #8eb47
1 Answer
Dec 14, 2014
The atomic mass units (amu) are a scale-set to 1/12th the mass of one atom of carbon-12.
They are what you would find as the "atomic mass" of an element on the periodic table.
example:
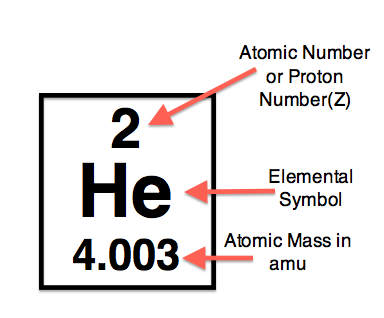
amu are extremely important because they help us to convert between mass and grams of an element or compound of elements in stoichiometry.
Rule: If you have one mole of any element you have a mass of the amu in grams.
example:

