How do you calculate the energy of an electron in the ground state of a hydrogen atom ?
1 Answer
Sep 27, 2015
You can calculate the ground state energy using The Bohr Model
Explanation:
A simple expression for the energy of an electron in the hydrogen atom is:
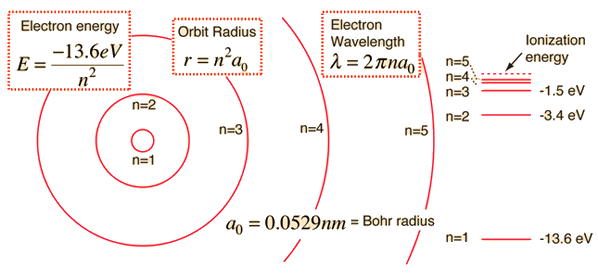
This gives rise to the familiar electron energy level diagram where they converge and coalesce.
So for an electron in
To convert to joules you can x this by
For many - electron atoms quite complicated approximate methods can be used which take into account factors such as electron interactions and screening effects.

