What is the difference between Arrhenius, Bronsted-Lowry, and Lewis acids and bases?
1 Answer
Arrhenius => release of
Bronsted-Lowry => donation/acceptance of proton
Lewis => acceptance/donation of electron pair
Explanation:
The definition of acid and base according to the three acid-base theories are:
Arrhenius acid-base theory
- acid - a substance produces hydrogen ions (
#H^+# ) in aqueous solution. - base - a substance that produces hydroxide ions (
#OH^-# ) in aqueous solution.
Here's the general equation defining Arrhenius acid and base:
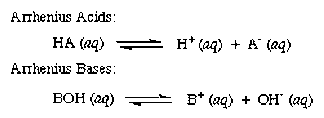
Bronsted-Lowry acid-base theory
-
acid - proton (hydrogen ion) donor
-
base - proton (hydrogen ion) acceptor
Here are examples showing general equation defining (a) acid and (b) base:
![http://biology.reachingfordreams.com/chemistry-cheat-sheet/chemical-equilibrium/42-the-nature-of-acids-and-bases]()
In (a), HA is an acid since it donates proton(
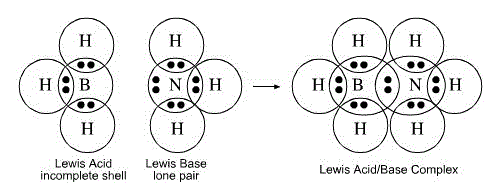
Lewis acid-base theory
-
acid - electron pair acceptor
-
base - electron pair donor
An example illustrating Lewis acid-base: