The #"Basicity"# of an acid refers to "the number of hydrogen atoms replaceable by a base" in one of its molecules.
#color(grey)("CH"_3)"COO"color(purple)("H")(aq)+"OH"^(-)(aq) to color(grey)("CH"_3)"COO"^(-)(aq)+color(purple)("H")-"O"-"H"(l)#
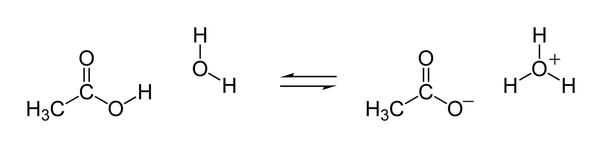
Ethanoic acids contain four hydrogen atoms; however, only the one directly bonded to the oxygen atom in the carboxyl #-"COO" color(purple)("H")# functional group at the end of the carbon chain participates in acid-base reactions. Removal of the rest three hydrogen atoms would render the carbon skeleton unstable and is thus energetically unfavorable (consider how polymers containers hold bases).

