How do the physical properties of soap allow water to be used to clean grease??
I don't understand because grease is non-polar and water is polar, how is water able to clean grease?
I don't understand because grease is non-polar and water is polar, how is water able to clean grease?
1 Answer
Soap molecules have two different properties - hydrophilic and hydrophobic components.
Explanation:
Soap is made of molecules that have very unique properties. The each have two 'ends'. One end likes water (hydrophilic) and thus enables the molecule to mix in water, the other end does not like water (hydrophobic) and so the molecule will always try to align itself so only the hydrophilic end is in contact with water.
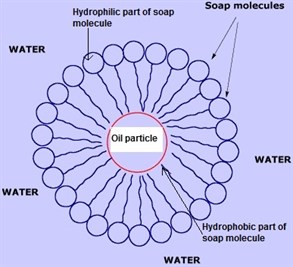
This allows a soap molecule to 'surround' a globule of grease or oil. The hydrophobic ends fall to face the grease and the hydrophilic ends face outwards to the water the soap is in. This effectively traps the grease but the fact that only hydrophilic parts of the molecule are 'on show' to the water allows the whole complex to dissolve in water, which means the grease can be 'carried' away in the water.
Interestingly, body cells exist by having the same properties as soap - a 'phospholipid bilayer';
)

