Question #b9657
2 Answers
The process of this Nucleophilic Acyl Subsctitution is explaied below

Heat helps in dissociation of water
Mechanism
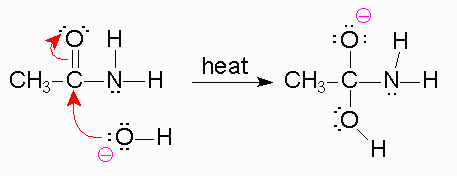
To begin, we know that in the first step, a strong base is going to be used like
Now, when the bond forms between the
 )
)
*The reason the amine is a poor leaving group is because the amine is a strong base, and strong bases are very unstable. Leaving groups are based off their stability in solution, like the halides, tosylate,
 )
)
Mechanism of acid catalysed amide hydrolysis
Step 1)An acid/base reaction. Since we only have a weak nucleophile and a poor electrophile we need to activate the ester. Protonation of the amide carbonyl makes it more electrophilic
Step 2:
The water O functions as the nucleophile attacking the electrophilic C in the C=O, with the electrons moving towards the oxonium ion, creating the tetrahedral intermediate.
Step 3:
An acid-base reaction. Deprotonate the oxygen that came from the water molecule.
Step 4:
An acid/base reaction. Need to make the -NH2 leave, but need to convert it into a good leaving group first by protonation.
Step 5:
Use the electrons of an adjacent oxygen to help "push out" the leaving group, a neutral ammonia molecule.
Step 6:
An acid/base reaction. Deprotonation of the oxonium ion reveals the carbonyl in the carboxylic acid product and regenerates the acid catalyst.

Coming to the main answer

At first though the formation of a sp3 hybridized carbon from a sp2 carbon leads to a less stable intermediate
https://chemistry.boisestate.edu/richardbanks/organic/amidehydrolysisbasictutorial3.htm
In the second step though the formation of a sp2 hybridized carbon from a sp3 carbon leads to a stabler intermediate,as the amine behaves as the leaving group this reaction is slower because amine is a poor leaving group.
It is unstable so the reaction will not favor it much,
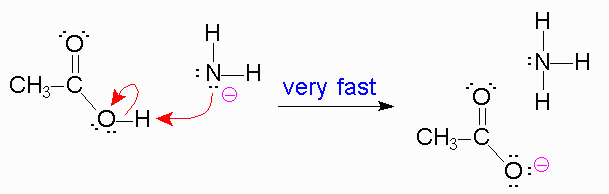
But the next reaction is fast because the unstable leaving group amine is stabilized by a proton from the reagent to form a nice, stable leaving group. So it is energetically favorable.

