If you heat a liquid and measure its boiling point, what are you measuring?
1 Answer
Apr 16, 2017
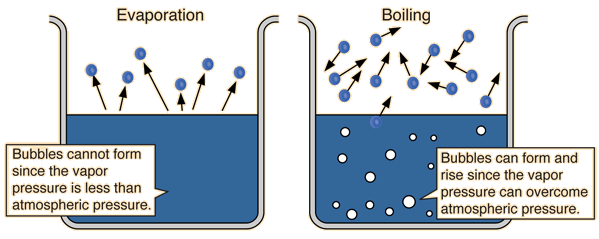
You are measuring the temperature necessary to make the liquid's vapor pressure equal to the atmospheric pressure.
Explanation:
Every liquid has a small amount of vapor over it. If the liquid does not boil, the vapor pressure is less than the atmospheric pressure. However, once the vapor pressure is equal to the atmospheric pressure, bubbles can form and create, what we know as boiling. The vapor pressure can be influenced by temperature, as when you increase the temperature of a liquid, more particles have enough energy for a phase change (from liquid to gaseous).
Hence, when measuring a liquid's boiling point you are measuring the temperature necessary to make the vapor pressure equal or greater to the atmospheric pressure.