How many valence electrons does helium have?
2 Answers
Explanation:
Helium is located in period 1, group 18 of the Periodic Table and has an atomic number equal to 2. As a result, neutral helium will only have 2 electrons surrounding its nucleus.
Valence electrons are the electrons located in an atom's outermost shell. In helium's case, both its electrons will be valence electrons.
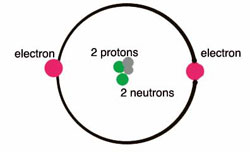
Its electron configuration can be used to better show this
Two electrons in its outermost (and only) shell, two valence electrons.
2 valence electrons
Explanation:
Helium only has two electrons, both of which fill its "s". It only has one electron shell so it has two electrons on the outside.



