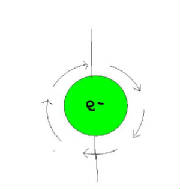
What two kinds of motion do electrons in an atom appear to have?
1 Answer
Apr 28, 2016
They are
Explanation:
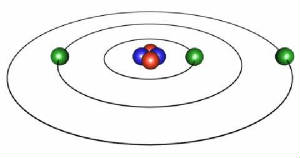
1) An electron revolving about the nucleus of an atom imparts a magnetic property on the atomic structure.
2)Electrons spinning on there on axis.
Opposite spins are designated as + and - signs.
Opposite directions tend to form pairs and neutralize their magnetic character.