Question #71543
1 Answer
Apr 19, 2015
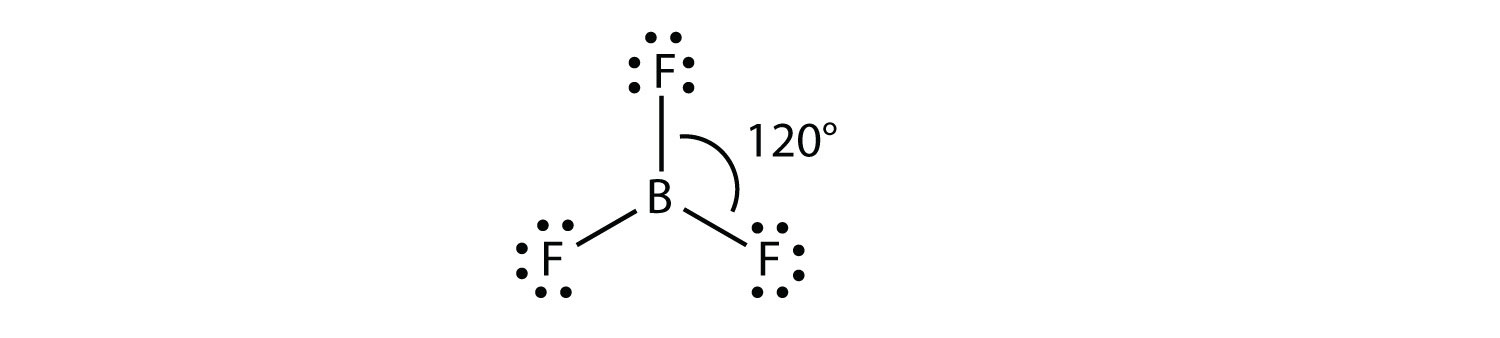
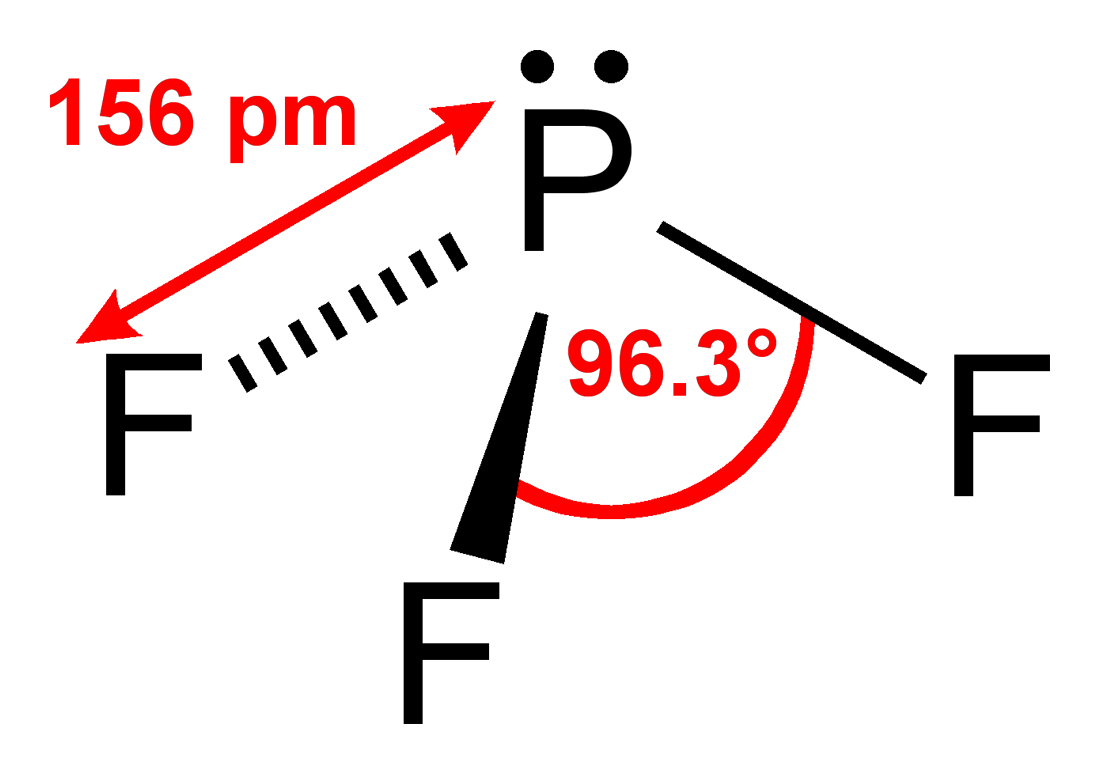
Because, boron has 3 valence electrons, and phosphor has 5 valence electrons. If we are talking about molecule with 3 chlorine atoms then all of boron valence electrons are included in covalent bond, also 3 phosphor valence electrons are included in covalent bond. We can conclude that phosphor has 2 free electrons (one electron pair).