Question #8855a
1 Answer
According to the chemistry textbook "Inorganic Chemistry" by Holleman-Wiberg, 2001, p. 527, sulfur hexabromide (
Normally we think of only unpaired valence electrons as bonding electrons, and the paired electrons as non-bonding electrons, However, electron orbitals can hybridize, forming hybridized orbitals from the s, p, and d orbitals.
In the case of sulfur hexabromide (
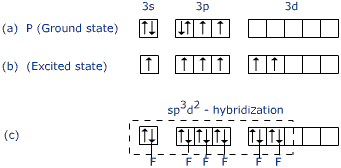
fig 1.27- Formation of
To account for the hexavalency in
These six orbitals get hybridised to form six sp3d2 hybrid orbitals. The name sp3d2 comes from the fact that the hybridized orbital comes from 1 s orbital, 3 p orbitals, and 2 d orbitals.
Each of these sp3d2 hybrid orbitals overlaps with a 2p orbital of a fluorine atom to form a S-F bond.
http://www.tutorvista.com/content/chemistry/chemistry-iv/atomic-structure/sp3d2-hybridization.php
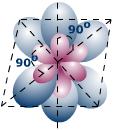
The six sp3d2 orbitals are at right angles to one another, and the molecule will have an octahedral shape.

