How do covalent bonds form?
1 Answer
According to Valence Bond Theory, a covalent bond forms when two valence orbitals, each with an unpaired electron, overlap, forming a covalent bond between the two atoms. Only orbitals with unpaired electrons will overlap and form a covalent bond.
In the following diagram, you will see two hydrogen (H) atoms, each with an electron configuration of
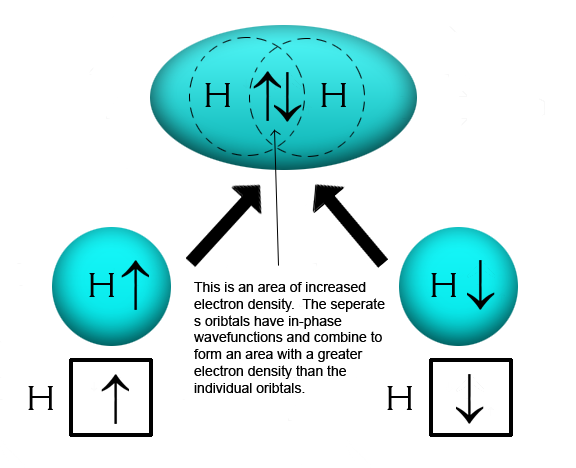
Helium has the electron configuration of

