#SO_2# is bent when you draw out the lewis dot structure (and a resonance structure):

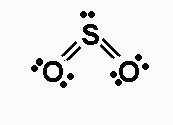
#SO_2# is asymmetrical with respect to the central S atom and so has a dipole. The electrons are not equally shared in the S-O bonds also, because O is more electronegative.






