Question #ee1c0
1 Answer
The general trend for period 3 oxides goes from basic, to amphoteric, to acidic as you move across from left to right.
This increase in acidity can be attributed to the change in the nature of the elements, which go from reactive metals, to poor metals, and finally to non-metals, and to the change in the nature of the bonds they form with oxygen, i.e. the difference in electronegativity (EN) between period 3 elements and oxygen.
Here's a table that describes this difference in EN between period 3 elements and oxygen:
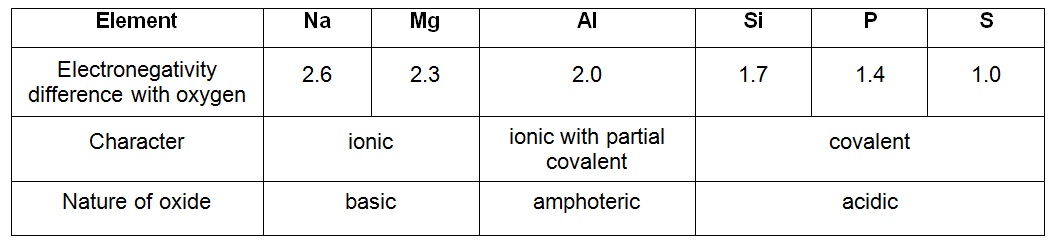
Sodium oxide and magnesium oxide are indeed basic, which implies that they form hydroxides when dissolved in water. They have the biggest EN difference with oxygen, which means that their bond will be predominatly ionic.
Things start to change with aluminum oxide,
The last four elements will form predominatly covalent bonds with oxygen, which will determine their acidic nature.
Another characteristic that increases acidity across period 3 oxides is the increasing oxidation state of the elements in their respective oxides. Here are the oxidation states for the highest oxides each period 3 element forms:
Again, increasing oxidation states matches the increasing covalent character of the oxide. So, as a conclusion
From left to right: decreased metallic character

